> Custom Services > Antibody Engineering > Leading Biology 2019-nCoV Antigen Reagents Facilitates to ReThe 2019-nCoV, is a contagious virus that causes severe respiratory disease, which is infectious. It’s known as the cause of the ongoing Wuhan coronavirus outbreak in December of 2019. Recent international reports have highlighted the emergence as there has been a growing number of cases identified outside of Hubei Province and internationally. nCoV Compared with the genetic sequence of virus with others, it appears to be a group 2B coronavirus, which is put in the same family as SARS and the genome of 2019-nCoV shares 89% nucleotide identity with SARS-related bat coronavirus. The phylogenetic trees of their orf1a/b, Spike, Envelope, Membrane and Nucleoprotein also clustered closely with those of the bat, civet and human SARS coronaviruses.
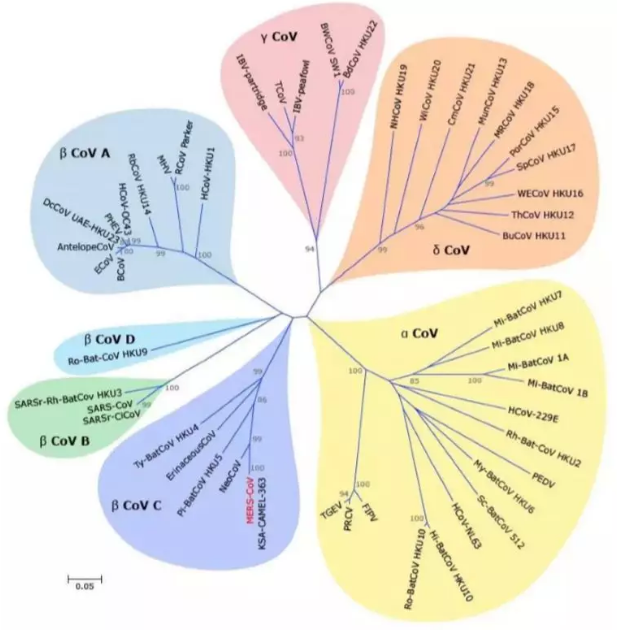
Genomic characterization of 2019-nCoV
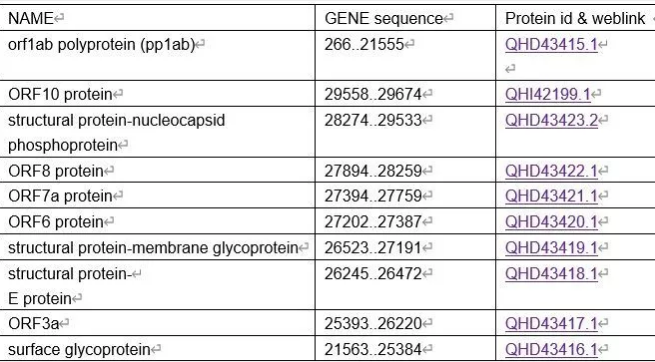
The 2019-nCoV is a large sized virus (approximately 120 nm in diameter). Genomic sequencing has shown that it is a positive-sense, single-stranded RNA coronavirus. Its single-stranded RNA genome is 29891 nucleotides in size, encoding 9860 amino acids, containing two flanking untranslated regions (UTRs) and a single long open reading frame encoding a polyprotein. The 2019-nCoV genome is arranged in the order of 5′-replicase (orf1/ab)-structural proteins [Spike (S)-Envelope (E)-Membrane (M)-Nucleocapsid (N)]−3′ and lacks the hemagglutinin-esterase gene.
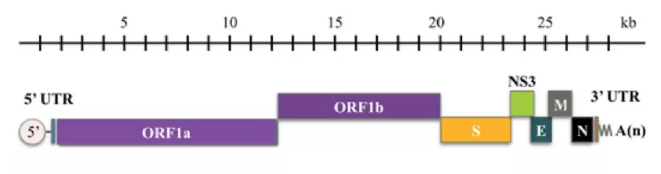
Diagnostic Solutions for 2019-nCoV
As 2019-nCoV and SARS N proteins have 90% homologous similarities, it is allowed that antibodies and antigens to SARS N protein to be used for diagnostic testing for 2019-nCoV. Diagnostic testing for the novel coronavirus 2019-nCoV is undertaken with 2 main approaches: genome sequencing and real-time reverse transcriptase PCR. Sequencing was used primarily in the early days of the outbreak for initial identification of this novel virus and is largely a tool of viral discovery.
l rRT-PCR: Almost all diagnostic testing for nCoV is done using rRT-PCR. PCR mixes and enzymes are extensively used in fast, sensitive molecular tests. RNA was extracted and tested by real-time RT-PCR with 2019-nCoV–specific primers and probes. If two targets (open reading frame 1a or 1b, nucleocapsid protein) tested positive by specific real-time RT-PCR, the case would be considered to be laboratory-confirmed. A cycle threshold value (Ct-value) less than 37 was defined as a positive test, and a Ct-value of 40 or more was defined as a negative test. A medium load, defined as a Ct-value of 37 to less than 40, required confirmation by retesting. If the repeated Ct-value was less than 40 and an obvious peak was observed, or if the repeated Ct-value was less than 37, the retest was deemed positive. The genome was identified in samples of bronchoalveolar-lavage fluid from the patient by one of three methods: Sanger sequencing, Illumina sequencing, or nanopore sequencing. Respiratory specimens were inoculated in cells for viral isolation in enhanced biosafety laboratory 3 facilities at the China CDC.
l Gene synthesis:
As the virus has been identified, most sequencing is being undertaken to further research in order to characterize it and monitor for viral mutation. Advances in gene synthesis mean that subunit vaccines can be developed quickly from one or more antigen-presenting viral genes or peptide sequences, which circumvents the need for scientists to handle live and potentially dangerous pathogens, and eliminates the time needed to clone DNA fragments.
2019-nCoV Products
Leading Biology, with years of expertise in life science, now offers antigens and polyclonal antibodies to 2019-nCoV (including the nucleocapsid protein). All the products are manufactured under ISO 90001 conditions and are available for research use only (RUO). Use Leading Biology double-stranded DNA fragments to create synthetic proteins designed as reagents for vaccine research.
Custome Service for 2019-nCoV Protein & Antibody
We offer custom recombinant protein and antibody service, no matter whether you need protein made in bacteria, yeast or mammalian hosts such as rabbit, mice, sheep, ect. The scientific team members with expertise experience in life science will work together with you on your project, providing technical support, sharing you with the project progress and focused on comprehensive analyses to ensure that your protein is made according to your requests in our lab in California, USA. Our technical team will provide the one-stop protein solutions from gene synthesis, subcloning to protein expression & purification. Customers can send samples to Leading Biology for processing of protein expression. Our advanced facilities and platform will ensure convenience, cost-saving and reliability. Custom service also supports the choice of quantity, purity, His tag or tag-free on request. Order related to 2019-nCoV products will be entitled to priority shipping and preferred pricing.
| No | Headline | Click | Author | Date |
| 1 | ScFv Phage Library Construction Service | 1201 | Leading Biology | 2020-05-27 |
| 2 | ScFv Phage Library Screening Service | 768 | Leading Biology | 2020-05-25 |
| 3 | Antibody Optimization Service | 642 | Leading Biology | 2020-05-25 |
| 4 | FcR Binding Assay Services | 1246 | Leading Biology | 2020-05-22 |
| 5 | Recombinant Antibody (IgG) Production Services | 763 | Leading Biology | 2020-05-21 |
| 6 | Recombinant Antibody (scFv) Production Services | 973 | Leading Biology | 2020-05-20 |