> Custom Services > Antibody Engineering > Recombinant Antibody (scFv) Production Services
What is scFv Antibody?
A single-chain fragment variable (scFv) is a recombinant antibody and has great potential for use in many fields such as research, diagnostic and clinical applications. The molecular weight is approximately 28 kDa. It consists of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of 10-25 amino acids. Each VH and VL domain contains three complementarity determining regions (CDRs). CDRs are short amino acid sequences that vary greatly among antibody molecules and, thus, are responsible for generating the great diversity of antibody binding specificity. The combination of the CDRs of the VH plus the CDRs of the VL determines the binding specificity of any given antibody.
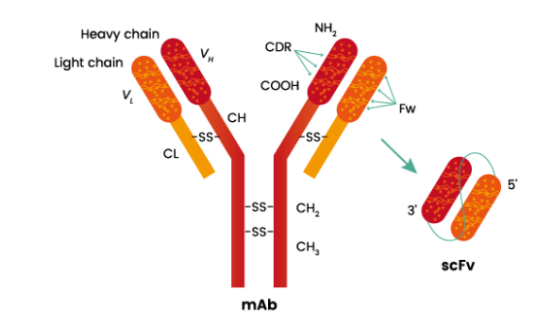
scFv Fragments Construction
To obtain scFv fragments, mRNA is first isolated from hybridoma (or spleen cells and B lymphocytes) followed by reverse transcribed into cDNA to serve as a template for the polymerase chain reaction (PCR) amplification of the antibody genes. With this method, large libraries with a diverse range of antibody VH and VL genes could be created. Using the techniques of affinity selection, the scFv fragment with the best affinity and specificity which is displayed on phage coat can be obtained.
In the scFv construction, the order of the domains can be either VH-linker-VL or VL-linker-VH. The specific antibodies produced from one selected hybridoma are essentially the same. When the antibody genes were successfully cloned and sequenced, the scFv fragments could be directly expressed in bacterial systems (i.e., E.coli) or mammalian systems (i.e., CHO or HEK293 cells).
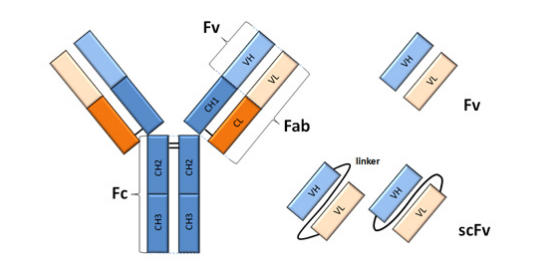
Cloning of Single-Chain Fv Fragments (scFv)
The Fv fragment of an antibody consists of a ~25 kDa heterodimer made of the VH and VL domains. The Fv fragment is the smallest fragment that holds a complete binding site of an antibody. Single-chain Fv fragments or scFv are obtained by connecting the VH and the VL domains by a linker in a single polypeptide.
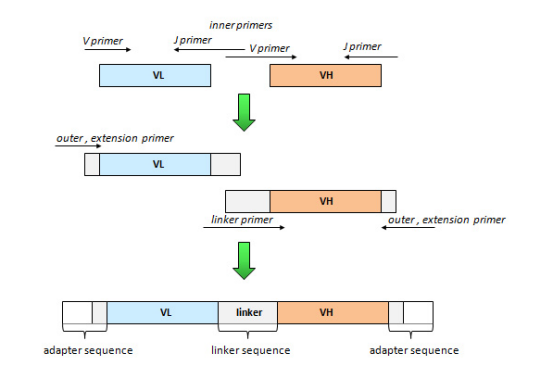
Cloning methodology
Cloning of the scFv is usually done by a two-step overlapping PCR (also known as Splicing by Overlap Extension or SOE-PCR). The VH and VL domains are first amplified and gel-purified and secondarily assembled in a single step of assembly PCR. The linker is generated either by overlap of the two inner primers or by adding a linker primer whose sequence covers the entire linker or more (three-fragment assembly PCR). The assembly PCR contains an equal amount of VH and VL domains, e.g. between 50 ng and 100 ng of each domain, and a lower amount of linker primer to prevent the preferential amplification of one domain, e.g. between one molar equivalent and 1/3 in weight of one domain. The outer primers during the assembly PCR can be either the primers that were used to amplify the domains or a new pair of extension primers; this last choice offers the possibility to extend the sequence on both sides of the scFv either to add restriction sites or to amplify the assembly independently of the domain sequence, an interesting situation when libraries are build. Some people do perform a few PCR cycles to assemble the two domains before adding the pair of outer primers, although the advantage of such a methodology is not clearly established.
Why Leading Biology?
With over 5 years of experience in custom antibodies, we offers numerous benefits for developing your custom antibody:
• Our industry leading titer guarantees of 1:50,000 eliminate the risk of not obtaining antibodies against peptide antigens.
• Our economies of scale allow us to pass cost savings to you and help maximize your budget.
• 100% of services are transparent throughout.
• Antibodies from our facility have been cited in many published research papers.
• By outsourcing antibody production and characterization needs to us, you can spend more time focused on your research.
• Our ideal location in California ensures that animals benefit from outdoor facilities and mild year-round temperatures.
• Confidentiality is a top priority for all projects. Antibodies that we manufacture belong to you and will not be commercialized.
| No | Headline | Click | Author | Date |
| 1 | ScFv Phage Library Construction Service | 1201 | Leading Biology | 2020-05-27 |
| 2 | ScFv Phage Library Screening Service | 769 | Leading Biology | 2020-05-25 |
| 3 | Antibody Optimization Service | 642 | Leading Biology | 2020-05-25 |
| 4 | FcR Binding Assay Services | 1247 | Leading Biology | 2020-05-22 |
| 5 | Recombinant Antibody (IgG) Production Services | 764 | Leading Biology | 2020-05-21 |
| 6 | Recombinant Antibody (scFv) Production Services | 973 | Leading Biology | 2020-05-20 |