> Custom Services > Antibody Engineering > Recombinant Antibody (IgG) Production ServicesRecombinant antibodies are produced in vitro by cloning antibody genes for immune-specific heavy and light antibody chains into high-yield expression vectors. These vectors are then introduced into expression hosts (eg bacteria, yeast, or mammalian) to generate the recombinant monoclonal antibodies. Recombinant antibodies can be used wherever you would normally use a traditional monoclonal antibody.
Recombinant Antibody Expression
To mimic the behavior of a recombinant human IgG antibody (targeting a human protein) in humans the best, specificity-matching recombinant mouse IgG antibodies derived from phage display mouse scFv / Fab libraries (targeting the mouse protein) are frequently produced and used in animal models. We use a modified vector to produce recombinant antibodies in HEK293 or CHO cells for transient or stable expression. Recombinant antibody service will offer antibodies with >95% purity and a QC report. The service covers a wide range of research needs from synthesis of a gene to highly purified antibodies within duration of as less as 6 weeks.
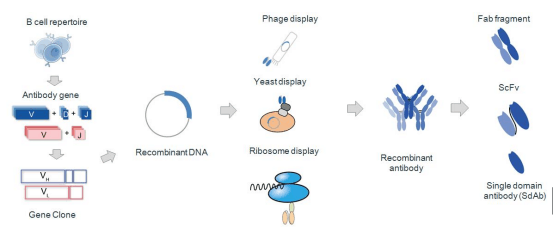
Production of Recombinant Antibody
How is recombinant antibody produced? Let me show you the process of recombinant antibody production. As we know, antibody is composed of two heavy chains (VH) and two light chains (VL).
Step 1: Variable genes of heavy chain and light chain antibody should be cloned by PCR and designed primers. By the recombinant DNA technology, link purified genes of VH and VL with prokaryotic expression vector which requires select in advance based on your target recombinant antibody fragments. If you need scfv fragment antibody, linker must be designed in your PCR strategy.
Step 2: Transformation: Electroporate ligation product of VH and VL received in previous step into cloned expression host cells. In this step, it is very important that highly competent of commercial source be recommended to obtain high transformation efficiency.
Step 3: Choose appropriate antibody display technology, such as phage display (which can screening small antibody fragment to obtain large antibody library); ribosome display (which can get a library of a capacity of 1014 without limitation of transformation efficiency and acquire mutant antibody library); yeast display (which make human antibody expression more superior because that yeast expression system is similar with mammalian cells). According to these above procedures, some recombinant antibodies can be constructed successfully, such as scfv antibody fragments, fab antibody fragments, and single domain antibodies.
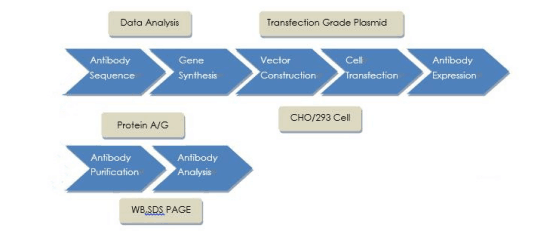
Service Procedures
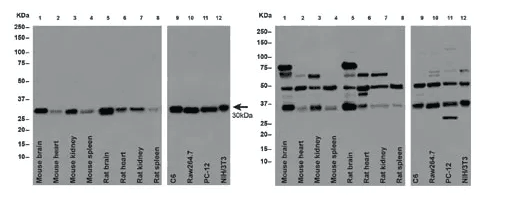
Application of Recombinant Antibody (rAb)
§ desire or requirement for a short circulating half-life in serum;
§ a smaller biologic that would have broader tissue distribution or the ability to penetrate tumors;
§ a molecule that can be manufactured in either yeast or E. coli to potentially reduce cost of goods or increase scale of manufacturing;
§ a molecule lacking an Fc effector functionality to eliminate both cellular responses against the target and potential for dimerization of receptors due to bivalency;
§ a bispecific antibody fragment, such as has been demonstrated by BiTEs (bispecific T cell engagers; diabodies and most recently, DARTs;
§ a molecule lacking an Fc effector functionality to eliminate both cellular responses against the target and potential for dimerization of receptors due to bivalency; a molecule that can be manufactured in either yeast or E. coli to potentially reduce cost of goods or increase scale of manufacturing.
Benefits of recombinant antibodies
Recombinant antibodies offer several advantages over both traditional monoclonal and polyclonal antibodies:
Improved consistency and reproducibility
Because recombinant antibodies are developed from a unique set of genes, antibody production is controlled and reliable. Several problems with hybridoma production can be avoided, such as gene loss, gene mutations, and cell-line drift. This leads to antibodies with very little batch-to-batch variability, giving you highly reproducible results.
With recombinant technology, it is easier to improve both antibody specificity and sensitivity through antibody engineering. The selection process for the desired clone occurs at both the hybridoma and recombinant cloning stages, allowing us to select the most favorable antibody qualities.
With the antibody genes isolated, antibody expression can be carried out at any scale and in a shorter timeframe than traditional monoclonal technology. This means we can generate tailored antibodies in weeks rather than months.
Once the antibody-producing gene is isolated, animal-free in vitro production can be implemented. For antibodies generated using our phage display technology, even the gene of the antibody can be isolated with an animal-free procedure.
Leading Biology provides recombinant antibody product, such as scfv antibody fragments, fab antibody fragments, single domain antibody, rabbit monoclonal antibodies, as well as recombinant antibody construction and expression services. If you are interested in learning more about how we can help with your projects, please don’t hesitate to contact us.
| No | Headline | Click | Author | Date |
| 1 | ScFv Phage Library Construction Service | 1201 | Leading Biology | 2020-05-27 |
| 2 | ScFv Phage Library Screening Service | 769 | Leading Biology | 2020-05-25 |
| 3 | Antibody Optimization Service | 642 | Leading Biology | 2020-05-25 |
| 4 | FcR Binding Assay Services | 1247 | Leading Biology | 2020-05-22 |
| 5 | Recombinant Antibody (IgG) Production Services | 763 | Leading Biology | 2020-05-21 |
| 6 | Recombinant Antibody (scFv) Production Services | 973 | Leading Biology | 2020-05-20 |