Humanization is important for reducing the immunogenicity of monoclonal antibodies derived from xenogeneic sources (commonly rodent) and for improving their activation of the human immune system. Since the development of the hybridoma technology, a large number of rodent monoclonal antibodies with specificity for antigens of therapeutic interest have been generated and characterized. Rodent antibodies are highly immunogenic in humans, which limits their clinical applications, especially when repeated administration is required. Importantly, they are rapidly removed from circulation and can cause systemic inflammatory effects as well.
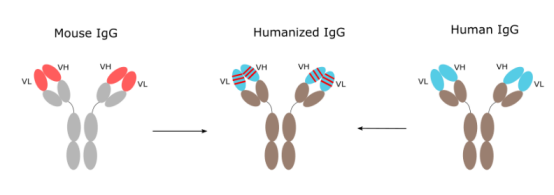
CDR-grafting
Humanization (also called Reshaping or CDR-grafting) is now a well-established technique for reducing the immunogenicity of monoclonal antibodies (mAbs) from xenogeneic sources (commonly rodent) and for improving their activation of the human immune system; in fact there are many humanised mAbs in clinical trials and a few have been given approval to be used as drugs. Although the mechanics of producing the engineered mAb using the techniques of molecular biology are relatively straightforward, simple grafting of the rodent complementarity-determining regions (CDRs) into human frameworks does not always reconstitute the binding affinity and specificity of the original mAb.
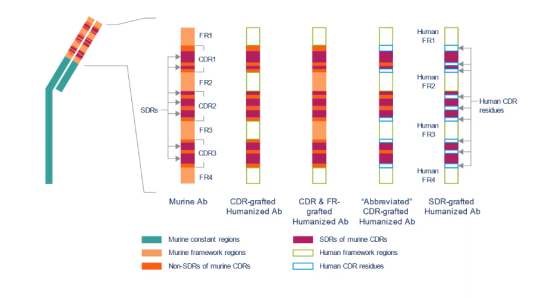
In order to humanize an antibody, the design of the humanized antibody is now the critical step in reproducing the function of the original molecule. This design includes various choices: the extents of the CDRs, the human frameworks to use and the substitution of residues from the rodent mAb into the human framework regions (backmutations). The positions of these backmutations have been identified principally by sequence/structural analysis or by analysis of an homology model of the variable regions' 3D structure.
Recently, phage libraries have been used to vary the amino acids at chosen positions. Similarly, many approaches have been used to choose the most appropriate human frameworks in which to graft the rodent CDRs. Early experiments used a limited subset of well-characterised human mAbs (often where the structure was available), irrespective of the sequence identity to the rodent mAb (the so-called fixed frameworks approach). Some groups use variable regions with high amino acid sequence identity to the rodent variable regions (homology matching or best-fit); others use consensus or germline sequences while still others select fragments of the framework sequences within each light or heavy chain variable region from several different human mAbs. There are also approaches to humanization developed which replace the surface rodent residues with the most common residues found in human mAbs ("resurfacing" or "veneering") and those which use differing definitions of the extents of the CDRs.
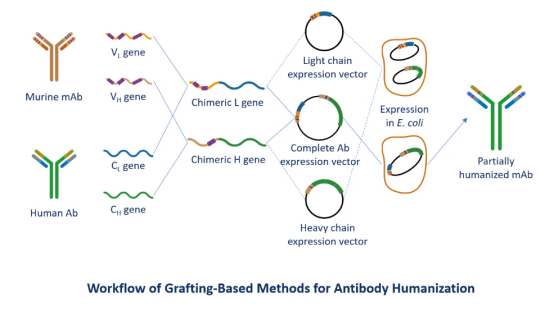
The General Procedures of Antibody Humanization
-- Production and characterization of the reference murine antibody or selection from a pre-made antibody library and determination of its affinity constant
-- Determination of the specific murine variable region sequences
-- Structural modeling of the mAb variable regions, construct of a panel of variants to be tested and optimization of antibody affinity
-- Affinity characterization and analysis of the humanized variants followed by recombinant expression in mammalian cells, such as CHO and 293T cells.
Service Features of Antibody Humanization Service
-- Advanced Algorithm for Aligning and Grafting of CDRs Affinity Guarantee
-- Antibody humanization from a broad range of parental species including mouse, rat, rabbit, llama and avian scFvs, Fabs and other antibodies humanized
-- A human antibody database and a proprietary method are used for analysis and design of humanized antibodies.
-- Flexible packages to work in synergy with your team: choose the number of humanized variants you would like to design from 9 to 25 combinations of VH and VL sequences.
If you want to get more information about this project and receive a customized proposal, please feel free to contact us at any time or click the link below for more antibody services:
https://www.leadingbiology.com/article_cat-18.html
| No | Headline | Click | Author | Date |
| 1 | ScFv Phage Library Construction Service | 1201 | Leading Biology | 2020-05-27 |
| 2 | ScFv Phage Library Screening Service | 769 | Leading Biology | 2020-05-25 |
| 3 | Antibody Optimization Service | 642 | Leading Biology | 2020-05-25 |
| 4 | FcR Binding Assay Services | 1246 | Leading Biology | 2020-05-22 |
| 5 | Recombinant Antibody (IgG) Production Services | 763 | Leading Biology | 2020-05-21 |
| 6 | Recombinant Antibody (scFv) Production Services | 973 | Leading Biology | 2020-05-20 |