> Custom Services > Antibody Engineering > Antibody Engineering and Modification TechnologiesAntibody engineering has become a well-developed discipline, including discovery methods, production strategies, and modification techniques that have led to clinical research and the sale of treatments. To achieve the long-term goal of producing monoclonal antibodies for all human beings, the clinical application of this effective drug category has been focused on in depth. However, antibodies are large molecules that pose many challenges in formulation, optimal pharmacokinetics, manufacturing, stability and process development. While further improvements in discovery techniques, such as phage display, ribosome display, and genetically modified animals, continue to improve our ability to quickly screen and optimize optimal binding molecules, antibody engineers have recently made more efforts to improve protein production and stability and engineered to improve biological properties in the monoclonal antibody effect. The second long-term goal of antibody engineering, the development of targeted drugs, has not yet been fully realized, but the apparent application of this antibody is currently being explored. Minimum binding proteins (such as Fab, scFv, and single variable domains) are the preferred target elements for some research drugs, while non-immunoglobulin stent proteins are explored as binding proteins in other designs. The need to utilize non-protein components in targeted drugs such as polymers, link agents and cytotoxics has led to the convergence of biocoupling chemistry and protein engineering in experimental antibody therapy.
Antibody engineering involves modifying the monoclonal antibody (mAb) sequence and/or structure to enhance or inhibit its function. Monoclonal abs have revolutionized the diagnosis and treatment of various diseases, particularly in cancer treatment. A challenging issue remains the production of therapeutic mAbs and Ab-derived drugs with the highest objective response rates and the lowest toxicity among patients[1-3]. The Ab Project is therefore a major translation research project designed to produce highly efficient mAbs with optimum processing, stability and tolerance [4].
Monoclonal Abs display a dual activity inherent to their structure:
· the variable region specifically binds one antigen
· the “fragment crystallizable” Fc in the constant region mediates effector functions, such as antibody-dependent cellular cytotoxicity (ADCC),antibody-dependent cellular phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC) [3].
Antibody Engineering Services
Leading Biology is a leading service provider focused on antibody engineering research, diagnosis and treatment. Our portfolio includes antibody affinity and maturation, improved antibody stability, chimeric antigen receptor (CAR) build, scFv/Fab build, and other antibody specialization and modification services.
Since the first hybrid tumor was acquired, there has been a possible modification of antibodies to enhance its function. During this decade, antibodies have become the main new drug for the treatment of a variety of diseases, including cancer and autoimmune diseases. As the basis for many diagnosis and treatment, the specific antigen binding ability of antibodies is essential for a variety of uses, such as expanding detection restrictions, extending separation half-life, reducing therapeutic doses, and improving overall efficacy. However, it is still beneficial to optimize the activity of these antibodies or antibody-related molecules relative to them.
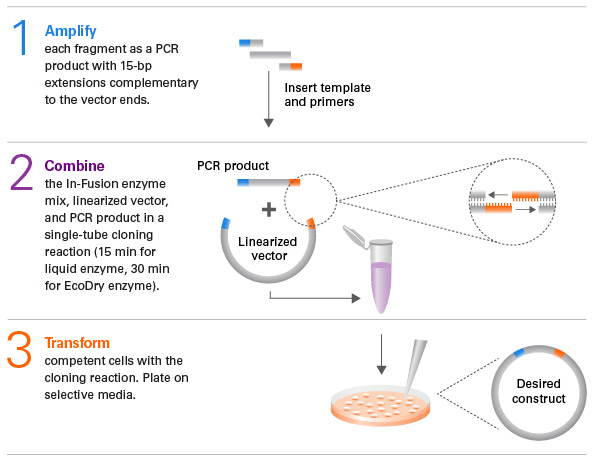
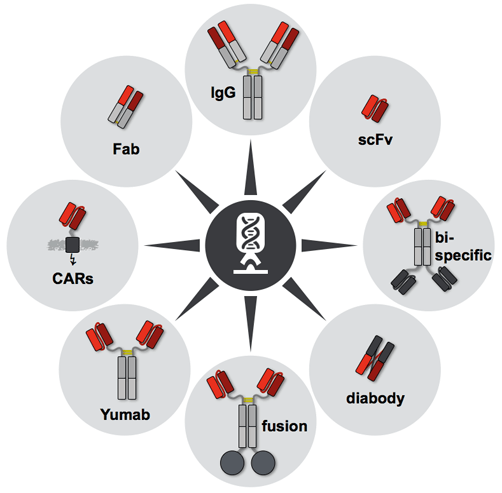
With modern molecular biology techniques, interested antibodies can be specifically modified or engineered to suit applications. If heavy and light chain sequences are determined, the antibodies of interest can be reconfigured into different or multiple formats (scFv, Fab, sdAb, bispecific antibodies, etc.) and other possible modifications, including affinity measurement, humanization, normalization, etc. One possible approach is to sequence and clone the area of interest of the adversary and port the selected area to the appropriate framework to build the plasmids. The predicted antibodies can then be produced by transient transfection through appropriate expression systems, such as mammalian cells and E. coli strains. As an option, antibody modification can also be combined with phage or other display methods to obtain a wider range of candidates or to select the product best suited for a variety of applications. It is worth noting that all of these modifications and manufacturing can be perfectly achieved by leading biological companies, who are recognized experts in antibody development and phage display technology.
Leading Biology has committed to the field of antibody engineering for over decade and established a series of comprehensive platforms to integrate various antibody engineering services. With the extensive experience, abundant industry knowledge and advanced technical skills, our scientists are fully confident in accomplishing even the most challenging antibody engineering projects.
Related Sections Services
· (Human) Antibody Camelization Services
· Generation of Bovine Antibodies with Ultralong CDR3s
· Monoclonal Antibody Caninization Service
· Monoclonal Antibody Murinization Service
· Simianization of Non-monkey Antibodies
· Chimeric IgG Construction
· Custom Cysteine Modification in Antibody
· Bispecific Antibody Services
· Bivalent and Bispecific ScFv/Fab
· Dual Variable Domain Immunoglobulin Production Service
References
1. Saeed A.F.U.H, et al. 2017. Antibody engineering for pursuing a healthier future. Front. Microbiol. 8:495.
2. Wang X. et al. 2018. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 9(1):63.
3. Quast I. et al. 2017. Regulation of antibody effector functions through IgG fc N-glycosylation. Cell. Mol. Life. Sci. 74(5):837.
4. Elgundi Z. et al. 2017. The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 122:2.
| No | Headline | Click | Author | Date |
| 1 | ScFv Phage Library Construction Service | 1202 | Leading Biology | 2020-05-27 |
| 2 | ScFv Phage Library Screening Service | 769 | Leading Biology | 2020-05-25 |
| 3 | Antibody Optimization Service | 643 | Leading Biology | 2020-05-25 |
| 4 | FcR Binding Assay Services | 1247 | Leading Biology | 2020-05-22 |
| 5 | Recombinant Antibody (IgG) Production Services | 764 | Leading Biology | 2020-05-21 |
| 6 | Recombinant Antibody (scFv) Production Services | 974 | Leading Biology | 2020-05-20 |