> Custom Services > Protein Related Services > Recombinant Protein Expression in Mammalian CellsApplication list of expression system
|
Type |
Escherichia coli |
Yeast cells |
Rod Virus - Insect Cells |
Mammal cells |
|
Expression system |
Prokaryotic |
Eukaryote |
Eukaryote |
Eukaryote |
|
Advantage |
Affordable; Fast; High yield; Widely used. |
Affordable; Fast; High yield; Partial post-translational modification |
Large gene capacity; Soluble protein; Suitable for toxic proteins; Translation modification similar to mammalian system |
Soluble proteins; Lower endotoxins; Better activity; Better post-translation modification; Instantaneous transfection and stable transfection expression |
|
Limitations |
Inclusion; No post-translation modification; Difficulty in expression of macromolecular weight proteins |
Non-human-derived Glycosylation; High-sugar dew retouching |
Long cycle High cost; Lack of partial glycolytitization |
Long cycles; |
|
Recommended Expressions |
Bacterial proteins; Antigens; Cytokines; Enzymes. |
Cytokines; Small molecular weight Proteins; Enzymes |
Cytoplasmic proteins; Toxic proteins; Transmembrane proteins; Secretion proteins; Kinases |
Secretion of protein; Transmembrane; Protein extracellular region; Recombinant antibody; Antibody fragment |
Overview of mammalian cell expression
Protein will have the function of folding and post-translational modification, bringing the protein closer to the native protein, thereby obtaining the same biological activity as the native protein.
Therefore, the mammalian cell expression system has the most extensive application in the development and production of recombinant protein drugs, especially therapeutic recombinant monoclonal antibodies.
Advantages of mammalian cell expression systems
Mammals are eukaryotic cells, and the organelle can fold proteins correctly.
Vectors can express recombinant proteins in mammalian cells with expressions up to 1-3 g/L
Efficient promoter control, stable expression, and a variety of expression system options:instantaneous expression, stable cell line expression, nutritional defect cell line expression (DHFR/GS), large-scale suspended cell expression, etc.
The most commonly used mammalian expression host cells (HEK293 and CHO) can be used to culture suspended cells produced within one month number of grams protein.
Leading biology mammalian cell expression system
Leading Biology provides one-step protein technical services, such as expression vector construction, transient transfection, expression testing, large-scale animal cell fermentation and protein purification.
|
Expression |
Cell line |
|
Transient expression |
HEK293, HEK293 cells were derived from 293 cell lines and cultured in serum-free suspension; CHO-S cells were clones isolated from Chinese hamster ovary (CHO K1) cells, and were tamed toculture in serum-free suspension. |
|
Stable expression |
CHO-S, CHO-K1 is derived from CHO. The culture condition of these cell lines are quite simple, and the cell adhesionstrength is moderate, thus it is easy to transfect. |
|
Construction of stable cell line |
DG44 cells (dihydrofolate reductase deficiency, DHFR) is derived from China hamster ovary (CHO) cells, it's commonly used for the construction of recombinant protein production cell line. The DG44 cell screening and co-amplification markers are DHFR genes. |
Expression vectors: common vectors pcDNA 3.1, pIRES, pTT3, pCEP4, pATX, etc. as well as our own patent vectors.
Leading biology mammalian cell expression service
1.Vector construction and optimization
1). cDNA can be cloned into the mammalian cell expression vector provided either by customers or by Leading biology;
2). The secretory signal peptide and purification tag can be added to the expression vector to promote the secretion expression of target protein and meet the needs of one step affinity purification;
3). Can produce and identify high efficiency and stable cell lines, which is suitable for large-scale fermentation.
2.Stable gene expression cells meet the needs of long-term survival
The entire experimental process provides long-term stable and adjustable protein production.
3.Transient expression (TGE)
1). Compared with the stable gene expression, TGE is mainly used for the preparation of recombinant protein in short term. It can be transfected rapidly in 10 days without the genetic selection of the plasmid DNA;
2). We using suspension cells for the instantaneous expression process, this method could achieve large-scale protein expression (-100L).
4.Process development and scale expansion
Leading biology is able to optimize the expression procedure and expression product, detection of cell expression, looking for the optimize fermenting condition, which is suitable for large-scale fermentation.
5.Purification
Leading biology has a complete protein purification system, have affinity purification, gel filtration, ion exchange and hydrophobicity chromatography purification methods. This system could fulfill protein purification both with or without tag and optimize the purification process.

Service Process
|
Service Items |
Time(weeks) |
|
Protein analysis and Codon optimization |
Before project start |
|
Gene synthesis |
2-4 |
|
Vector construction |
1 |
|
Gene expression and purification |
2 |
|
1L fermentation and purification |
2 |
|
Tag removal |
2 |
|
Large scalefermentation and purification |
According to the requirements of the project |
|
Total |
8-10 |
Classic case
A biologic organelles matrix protein
Full length 334aa,
Weight 37.03kDa, Fc tag protein,
293F cell expression.
After the coding gene whole codon optimization, it was constructed on a pTT3 vector, sequencing verification followed by 30mL test expression (Fig.1).
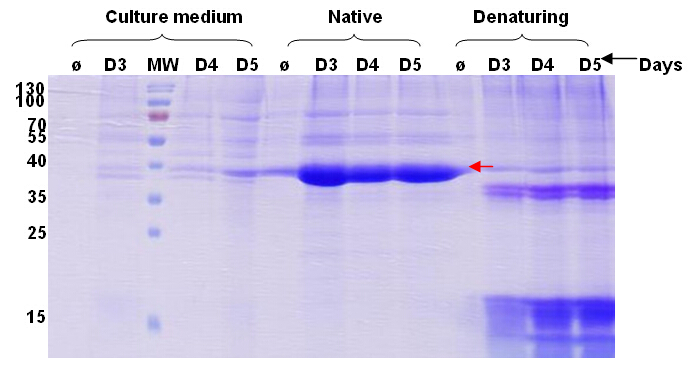
Figure 1. Expression result (Coomassie blue staining)
MW. Molecular weight marker. Ø. Non-transfected (negative control).
To get the maximum recovery of target protein, we did Protein G purification twice to the flow through (Fig.2, Fig. 3), the remaining target protein in FT can be observed after second purification.
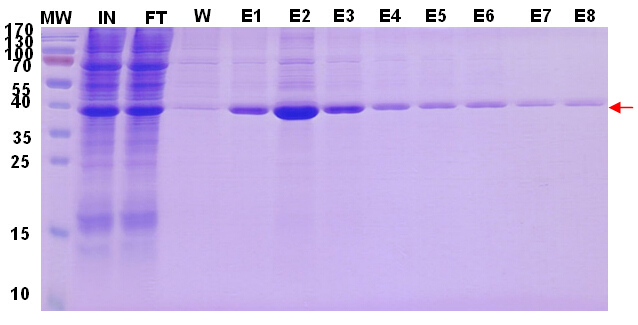
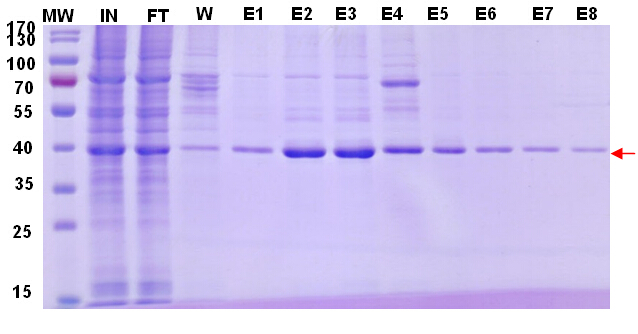
Figure 2. Target protein purification profile. Figure 3. Target protein purification profile.Reducing-PAGE analysis.
Therefor, we raised the salt concentration to 300mM, after elution, the amount of the remaining protein in FT decreased significantly (Fig. 4).
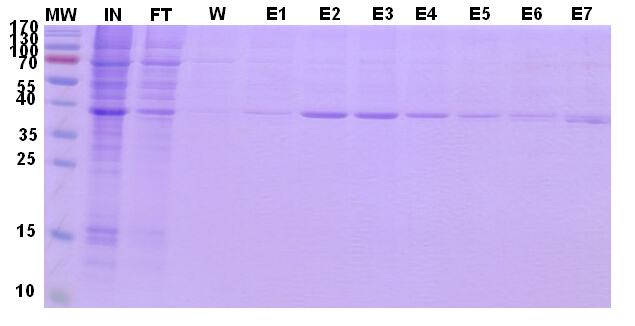
Figure 4. Target protein purification profile. Coomassie blue staining.Reducing-PAGE analysis.
After experimental conditions optimization, we do large-scale expression, using 2L fermentation.
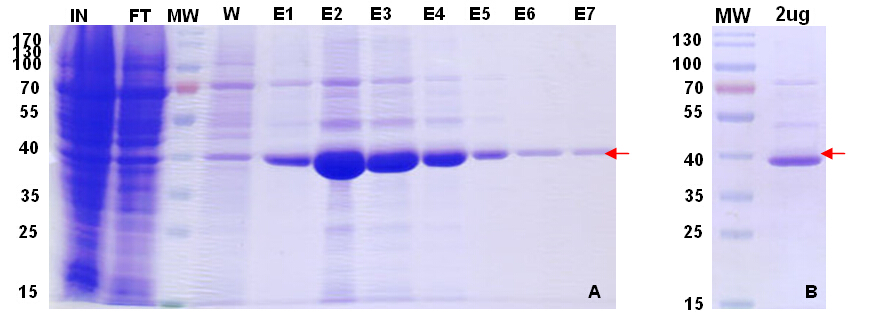
Figure 5. Target protein purification profile and QC gel.
Related Services
Protein Expression in Bacteria
Unique Protein Production Service
Protein Expression in Cell-Free System
Rich in Disulfide Bond Protein Expression
Protein Expression in Mammalian Cells
Protein Expression in Baculovirus
Protein Expression in Bacillus subtilis
Why Leading Biology?
At Leading Biology, we custom protein purification design for every single protein to ensure the production and recovery rate as high as possible.
Working with us, you will get stability, and it means a reliable partner to help streamline your R&D process.
Working with us, you will get the guaranteed service to accommodate your requirements.
- Innovative configurations of chromatography columns custom tailored to each protein
- Vigorous quality control system to ensure the required quality and reproducibility
- Production capacity of up to tens of grams
- Flexible scale-up protein production
- Competitive price with fast turnaround time
Contact Information
Please obtain a quote before ordering, and refer to the quote number when you place an order.
Orders are typically confirmed within 12 hours.
Have a Question? Email us info@leadingbiology.com
Order Products: Order Related Products
By Phone: 1-661-524(LBI)-0262 (USA)
| No | Headline | Click | Author | Date |
| 1 | Ubiquitination | 1489 | Leading Biology | 2020-06-24 |
| 2 | Acetylation | 1048 | Leading Biology | 2020-06-23 |
| 3 | Single Molecule Protein Detection | 1289 | Leading Biology | 2020-06-22 |
| 4 | Phosphorylation | 1288 | Leading Biology | 2020-06-22 |
| 5 | Methylation | 1482 | Leading Biology | 2020-06-22 |
| 6 | Protein Detection and Immunoassay | 1429 | Leading Biology | 2020-06-17 |