Introduction
Escherichia coli (E. coli) protein expression system is one of the most widely used system to produce recombinant proteins in recent decades. After developed and improved continuously by scientists for years, E. coli expression system is being able to express a wide variety of different types of recombinant proteins in many fields, even proteins that contain complex structures. Two features that make E. coli highly useful in lab research field are its rapid reproduction ability and its generation of clones, or genetically identical bacteria. This system is applied to prepare antigens, ligands, cytokines, bacterial proteins and so on. Leading Biology’s E.coli protein expression system serves you with a simple structure, clear genetic background, short culturing period and low culture cost.
Escherichia coli expression system
1. Clearer mechanism of metabolic pathways and gene expression
Through whole-genome sequencing, there are total 4,405 open reading frames;
Mature gene cloning and expression system;
Rapid propagation, simple culture method, easy to operate and stable inheritance.
2. Short life cycle
Escherichia coli has a strong reproductive ability. It could reproduce every 20-30 minutes under adequate nutritional conditions;
Cost-effective for large scale fermentation and enormous potential for survival.
3. High level of target gene expression
The expression level is higher than normal eukaryotic expression systems. It could express up to5%-30% of total protein for some exogenous genes.
4. Clear genetic background
The genetic background and physiological property of Escherichia coli is quite clear. There are multiple bacterial strains of different drug resistance, auxotrophic mutation and suppression mutation to select.
5. Strong anti-pollution capacity, simple downstream process and easier to control.
6. There are plenty of existing expression vectors to select, and it’s an FDA approved genetic engineering host strain.
Advantages of E.coli Expression Service
1. The successful rate of E.coli soluble expression is as high as 95%.
2. A variety of E. coli expression vectors are available, including pET28b, pET22b, pGEX-6P-1, etc.
3. A variety of E.coli expression hosts are available, including the standard expression strain BL21, strain T7E which could increase the expression of protein, the strain C41 which could express the toxic protein, the ultra-low temperature strain Arctic which could enhance soluble protein expression, and other expression strains modified by our professional teams, etc. We can meet the different needs of customers.
4. Optimization of the expression system. The optimization of the expression system can solve the problems of non-expressed proteins and inclusion bodies, such as compatibility test of expression vector and expression strain, expression condition optimization, protein renaturation and so on.
Service Package
|
Service Items |
Time(weeks) |
|
Protein analysisand codon optimization |
Before project starts |
|
Gene synthesis |
2-4 |
|
Vector construction |
1 |
|
Gene expression and purification |
2 |
|
1L fermentation and purification |
2 |
|
Tag removal |
2 |
|
Large scale fermentation and purification |
According to the requirements of the project |
|
Total |
8-10 |
Notes:
1. The expression and purification test including 2 vectors, 3 expression strains, 2 expression temperatures, 12 condition tests, according to preliminary experiment results, the test cycle can be changed.
2. The alternative optimization conditions we provide are as follows:
|
Expression vector |
pET28b\pET32a\pGEX-6p-1\pET22b\pBAD\pTrcHis etc. |
|
Expressioncell line |
Arctic\BL21(DE3)\Origami\Rosetta\T7 Expression |
|
Expression temperature |
16C\30C\37C |
Classic case
For a prokaryotic protein:
1. Expression system optimization, including host bacteria, induction temperature, inductiontime, inducer concentration and culture medium.
|
Host bacteria |
Induction temperature |
Induction time |
Inducer |
Culture medium |
|
T7E, BL21, C41, Arctic |
30C/37C |
1h/4h |
IPTGself-induction |
LB/Customized/Isotope/Auto-induction |
After 3 rounds of optimization, we chose BL21+pGEX-6p-1 combination, and found that temperature has the greatest influence on the expression of target protein (Fig. 1).
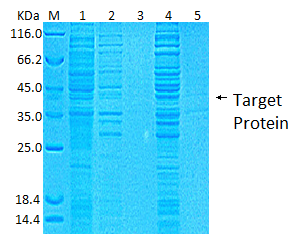
Fig. 1: Small scale fusion protein SDS-PAGE M: Protein Marker; 1: Total protein before induction; 2: 20C supernatant; 3: 20C precipitate; 4: 37C supernatant: 5: 37C precipitate
2. After determining the optimal expression conditions, we use GST Agarose affinity chromatography to purify proteins (Fig. 2).
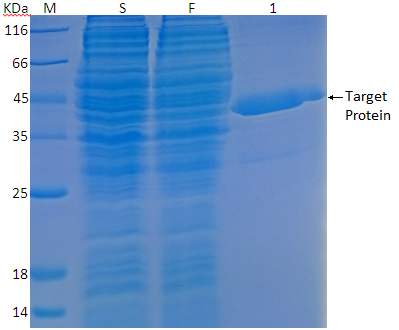
Fig. 2: GST Agarose affinity chromatography purification SDS-PAGE M: Protein marker; S: Loading sample; F: Outflow sample; 1: 20mM GSH Elution component;
3. Under the condition that the purification efficiency is acceptable, we do large-scale fermentation of the protein, and do SDS-PAGE test, in figure 3, after purification of the fusion protein, SDS-PAGE showed a clear band at the appropriate position, indicate that the protein has successfully purified.
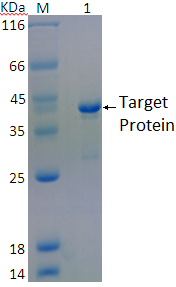
Fig. 3: Protein purification SDS-PAGE M: Protein marker; 1: Target protein
4. Protein concentration determination
Using SK3071 non-interference protein assay kit test protein concentration is 1.06mg/mL, the volume of the sample to be measured is 10μL, The result is as follows:
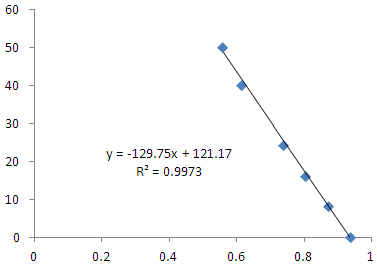
|
Test 1 |
Test2 |
Average |
BSA (μg) |
|
0.939 |
0.939 |
0.939 |
0 |
|
0.875 |
0.874 |
0.875 |
8 |
|
0.808 |
0.804 |
0.806 |
16 |
|
0.740 |
0.746 |
0.743 |
24 |
|
0.618 |
0.616 |
0.617 |
40 |
|
0.855 |
0.849 |
0.852 |
50 |
|
0.891 |
0.878 |
0.885 |
Target protein |
Fig. 4 Protein concentration detection
Related Services
Protein Expression in Bacteria
Unique Protein Production Service
Protein Expression in Cell-Free System
Rich in Disulfide Bond Protein Expression
Protein Expression in Mammalian Cells
Protein Expression in Baculovirus
Protein Expression in Bacillus subtilis
Why Leading Biology?
At Leading Biology, we custom protein purification design for every single protein to ensure the production and recovery rate as high as possible.
Working with us, you will get stability, and it means a reliable partner to help streamline your R&D process.
Working with us, you will get the guaranteed service to accommodate your requirements.
- Innovative configurations of chromatography columns custom tailored to each protein
- Vigorous quality control system to ensure the required quality and reproducibility
- Production capacity of up to tens of grams
- Flexible scale-up protein production
- Competitive price with fast turnaround time
Contact Information
Please obtain a quote before ordering, and refer to the quote number when you place an order.
Orders are typically confirmed within 12 hours.
Have a Question? Email us info@leadingbiology.com
Order Products: Order Related Products
By Phone: 1-661-524(LBI)-0262 (USA)
| No | Headline | Click | Author | Date |
| 1 | Ubiquitination | 1231 | Leading Biology | 2020-06-24 |
| 2 | Acetylation | 816 | Leading Biology | 2020-06-23 |
| 3 | Single Molecule Protein Detection | 1038 | Leading Biology | 2020-06-22 |
| 4 | Phosphorylation | 1064 | Leading Biology | 2020-06-22 |
| 5 | Methylation | 1146 | Leading Biology | 2020-06-22 |
| 6 | Protein Detection and Immunoassay | 1170 | Leading Biology | 2020-06-17 |